Abstract
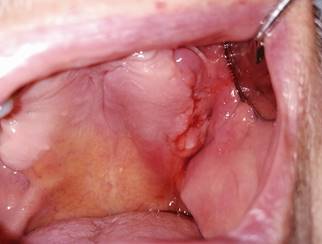
A 78-year-old patient seeks care for unresolved bleeding on the left maxillary alveolar ridge. He had a history of bone metastasis treated with IV zoledronic acid, which was discontinued after medical discharge and previous surgical treatment of osteonecrosis of the jaws in the affected site. Continuity disruption with inflammation at the bone ridge was observed, with blood, purulent, and necrotic exudate. The radiograph showed diffuse and osteolytic radiolucency of the affected area, and necrotic tissue was detected at a microscopic level. The affected area was rinsed with 0.12% chlorhexidine, 400 mg amoxicillin/clavulanic acid and pentoxifylline was administered every 12 hours, and tocopherol 1000 IU every 24 hours. The area was evaluated after a month. Antibiotic therapy was discontinued, and the patient continued to be monitored every two weeks. At six months, there is complete resolution, and mucosal and bone tissue healed without recurrence.
References
Heifetz-Li JJ, Abdelsamie S, Campbell CB, Roth S, Fielding AF, Mulligan JP. Systematic review of the use of pentoxifylline and tocopherol for the treatment of medication-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;128(5):491-497.e2
Govaerts D, Piccart F, Ockerman A, Coropciuc R, Politis C, Jacobs R. Adjuvant therapies for MRONJ: A systematic review. Bone. 2020;141(115676):115676.
Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 2014;72(10):1938–56.
Sivolella S, Lumachi F, Stellini E, Favero L. Denosumab and anti-angiogenetic drug-related osteonecrosis of the jaw: an uncommon but potentially severe disease. Anticancer Res. 2013;33(5):1793–7.
Pakosch D, Papadimas D, Munding J, Kawa D, Kriwalsky MS. Osteonecrosis of the mandible due to anti-angiogenic agent, bevacizumab. Oral Maxillofac Surg. 2013;17(4):303–6.
Foncea C, von Bischhoffshausen K, Teuber C, Ramírez H, Goñi I, Sánchez C, et al. Osteonecrosis of the jaws. Rev Med Chil. 2020;148(7):983–91
Beth-Tasdogan NH, Mayer B, Hussein H, Zolk O. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst Rev. 2017;10:CD012432.
Martos-Fernández M, Saez-Barba M, López-López J, Estrugo-Devesa A, Balibrea-Del-Castillo JM, Bescós-Atín C. Pentoxifylline, tocopherol, and clodronate for the treatment of mandibular osteoradionecrosis: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(5):431–9.
Seo MH, Eo MY, Myoung H, Kim SM, Lee JH. The effects of pentoxifylline and tocopherol in jaw osteomyelitis. J Korean Assoc Oral Maxillofac Surg. 2020;46(1):19–27.
Lyons AJ, Brennan PA. Pentoxifylline – a review of its use in osteoradionecrosis. Br J Oral Maxillofac Surg. 2017;55(3):230–4.
Cavalcante RC, Tomasetti G. Pentoxifylline and tocopherol protocol to treat medication-related osteonecrosis of the jaw: A systematic literature review. J Craniomaxillofac Surg. 2020;48(11):1080–6.
Owosho AA, Estilo CL, Huryn JM, Yom SK. Pentoxifylline and tocopherol in the management of cancer patients with medication-related osteonecrosis of the jaw: an observational retrospective study of initial case series. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(4):455–9.
Epstein MS, Wicknick FW, Epstein JB, Berenson JR, Gorsky M. Management of bisphosphonate-associated osteonecrosis: pentoxifylline and tocopherol in addition to antimicrobial therapy. An initial case series. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(5):593–6.
Magremanne M, Reychler H. Pentoxifylline and tocopherol in the treatment of yearly zoledronic acid-related osteonecrosis of the jaw in a corticosteroid-induced osteoporosis. J Oral Maxillofac Surg. 2014;72(2):334–7.
Hayashi M, Pellecer M, Chung E, Sung E. The efficacy of pentoxifylline/tocopherol combination in the treatment of osteoradionecrosis: Pentoxifylline/tocopherol combination. Spec Care Dentist. 2015;35(6):268–71.

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.